
Figure 1
A REVISED APPROACH IN WRITING LEWIS STRUCTURES
Wan-Yaacob Ahmad and Mat B. Zakaria*
*E-mail: drmbzak@ukm.my
School of Chemical Sciences and Food Technology
Universiti Kebangsaan Malaysia
43600 UKM Bangi, MALAYSIA
ABSTRACT
Each charge of a simple polyatomic ion is assign to a surrounding oxygen followed by nitrogen and sulfur, or to a central atom for the one only with surrounding hydrogen or halogens. Typical covalent bonds for surrounding atoms are written to a central atom of a simple polyatomic ion or molecule. It is followed by an assignment of lone pairs on the central atom to produce a structure. Most multiple-bonded structures should be modified to generate single Lewis structure or resonance structures. This approach also can be applied for larger polyatomic covalent species.
INTRODUCTION
Recently, we published an article on how to derive Lewis structures for polyatomic molecules and ions using a direct electron pairing approach.1 Re-examination of the article has led to a method where the writing of surrounding atom Lewis structures can be omitted. This is made easy by the fact that there are only eight common surrounding atoms, i.e. the halogens (F, Cl, Br, I = X), H, chalcogens (O, S) and N, which are situated at the upper right corner of the periodic table. In the previous article, a resonance structure was termed as the one with an allylic, propargylic, or allenic moiety.1 It is now redefined as a structure with a central atom consists of an incomplete octet of six electrons or an octet where there is a multiple bond on one surrounding atom and at least a lone pair on other(s). The structure is confined only to the one having a steric two or three central atom. The steps for writing the Lewis structures are given below.
STEP 1: CHARGE DISTRIBUTION ON ATOMS
For a polyatomic ion, place each charge on each surrounding O (followed by N, and S) or solely on a central atom for the one only with surrounding H/X. This is to avoid forming non-covalent monatomic O2-
/H+
/H-
/X-
ions or double-bonded surrounding X+
atoms. A negative charge on the most electronegative polyvalent O or N as a surrounding atom is approriate because this will stabilize the structure for a polyatomic anion. In the previous article, the number of valence electrons on a neutral central atom and the neutral surrounding-atom Lewis structures were firstly written.1 The charge was then distributed on the corresponding atoms together with altering the number of electrons. The placement of a positive charge on a central atom1 will give rise to a structure similar to the one assigned to the surrounding O. For neutral molecules, one could proceed to the step 2.
STEP 2: FORMATION OF TYPICAL BONDS FOR SURROUNDING ATOMS
Write a single bond on a central atom for surrounding H/X/O-
/S-
, a double bond for surrounding O/S/N-
, and a triple bond for surrounding N/O+
. In the previous article, the unpaired electrons on surrounding atoms were shared with valence electrons from the central atom, albeit mentally, to form two-electron bonds.1 This is carried out after the surrounding H/X/O-
/S-
, O/S/N-
, and N/O+
Lewis structures with one, two, and three unpaired electrons were written as in Figure 1.

Figure 1
After sharing, the respective atom surely produces a single, double, and triple bond as in Figure 2.

Figure 2
The writing will be simplified if lone pairs on the respective surrounding atoms are omitted as in Figure 3.

Figure 3
Except for H, it is inferred that each of the above atom in Figure 3 has an octet of electrons. Therefore, one covalent bond involves three lone pairs, two covalent bonds involve two lone pairs and three covalent bond involve one lone pair. Apparently, the structure for a simple covalent species can be derived without the need of writing surrounding atom Lewis structures. A list of the above surrounding atoms and the type of bonds each formed, is shown in Table 1 by referring to atoms in the outer position in the second column.
Table 1: Atoms commonly occurred in polyatomic molecules and ions with the type and number of covalent bonds each formed.
|
Atoms |
Outer Position |
Inner Position |
Number of lone pairs |
|
Types of covalent bonds |
Number of covalent bonds |
||
|
H, X, O- , S- |
single |
- |
3 |
|
O, S, N- |
double |
2 |
2 |
|
N, O+ |
triple |
3 |
1 |
|
C |
- |
4 |
0 |
STEP 3: NUMBER OF LONE PAIRS ON CENTRAL ATOM
Sum the number of bonds on a central atom since it represents the number of its
"evenly" shared electrons; the remaining valence electrons, i.e. the number of valence electrons that is not involved in bonding, if any, will become lone pairs on the central atom. A completely single bonded structure represents a single Lewis structure.
STEP 4: MODIFICATION OF MULTIPLE-BONDED STRUCTURES
Some multiple-bonded structures need further transformation. A multiple bonded steric four or beyond structure should be converted to a completely single bonded structure in which every p
bond is converted to a lone pair on a surrounding atom.2,3 Formal charge on the central atom increases by one positive unit. A steric two or three structure with a central atom consists of an incomplete octet of six electrons or an octet where there is a multiple bond on one surrounding atom and lone pair(s) on the other(s) (BO2-, CO2, FNO, etc.) is one of the resonance structures for a species. A multiple bonded steric two or three structure with a central atom consists of an expanded octet (N2O, NO2+, SO3, etc.) can be reduced to one of the resonance structures by converting enough p
bond(s) to lone pair(s) on surrounding atom(s). Again this structure can be converted to one or two other resonance structures. It involves the conversion of a lone pair on one surrounding atom to a p
bond and the other p
bond to a lone pair on another surrounding atom. Formal charge on central atom does not change. Note that a steric two or three structure with a central atom consists of an octet where there is a multiple bond on one surrounding N/O but the others involve H (as in HCN, HCO+
, or H2CO) is not one of the resonance structures for a species. It is purely a single Lewis structure.
PRODUCING LEWIS STRUCTURES
To illustrate the proposed procedure, specific examples are given below.
Example 1: I3-
A minus for the triiodide ion should not be assigned to the surrounding I (step 1). Instead it is placed on the central I. Therefore, I3- equals II-
I. Each surrounding I, in II-
I, forms a single bond (step 2) to give structure 1. The two bonds central I-
in 1 with a total of eight valence electrons utilised two electrons for bonding. The six remaining electrons formed three lone pairs as shown is structure 2 (step 3). An all single-bonded structure 2, where the central I-
consists of an expanded octet of ten electrons, is a Lewis structure for the triiodide ion.

Example 2: XeO64-
Each of the four minus from the perxenate ion is assigned to each of the first four O whereas the other two are neutral. Hence, XeO64- equals XeO-O-O-O-OO (step 1). Each surrounding O- in XeO-O-O-O-OO forms a single bond while each surrounding O yields a double bond as in structure 3 (step 2). The central atom Xe in structure 3 with eight valence electrons contributes all for sharing to form eight covalent bonds. The central Xe is without lone pair (step 3). A two double bonded steric six structure 3 can be reduced to a completely single bonded Lewis structure by converting every p bond in every Xe=
O double bond to a lone pair on O, thus yielding structure 4 (step 4). The central Xe in structure 4 consists of an expanded octet of twelve electrons from six Xe(2+)-O(1-) bonds.

Example 3: (NCS)-
A minus for the species is assigned to the surrounding N. So, (NCS)-
equals N-CS (step 1). The respective N-
and S, in N-CS, yields a double bond as in structure 5 (step2). A central C in structure 5 with four valence electrons contributes all for sharing to form four bonds (step 3). A steric two structure 5 with two double bonds and a central C consists of an octet is one of the resonance structures for (NCS)- since the surrounding N-
or S possesses two lone pairs. Conversion of a lone pair on surrounding N-
in structure 5 to a p
bond and the other p
bond to a lone pair on surrounding S gives rise to resonance structure 6 (step 4). Structure 5 also can be converted to resonance structure 7 by a similar but reverse manner. Structures 5, 6, and 7 are three resonance structures for thiocyanate ion, and they are inter-convertible.
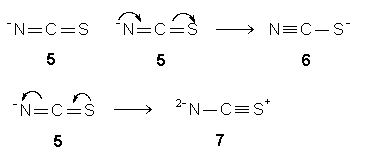
Example 4: O3
Each surrounding O in OOO for the neutral O3 forms a double bond to give structure 8 (step 2). A central O in structure 8 with six valence electrons utilises four of them to form four bonds whereas the remaining two exists as a lone pair as shown in structure 9 (step 3). A two double bonded steric three structure 9 achieves an octet central O after its right double O=
O bond is converted to a single O(1+)-O(1-) bond as in structure 10 (step 4). It is one of the resonance structures for an ozone molecule. A double bond in structure 10 cannot be converted to a single bond because it would lead to a central O with an incomplete octet of six electrons. The resonance structure 10 gives another resonance structure 11, and both are inter-convertible.

LEWIS STRUCTURES FOR LARGER POLYATOMIC SPECIES
The proposed procedures for writing Lewis structures also can be extended to larger inorganic polyatomic molecules or ions. This is because each H/X/O-/S- always occupies the outer position in any structure forming a single bond. An O/S/N-, on the other hand, either occupies the outer position in a structure with a double bond or occupies the inner position in a structure with two single bonds. A N/O+ resides at the outer position in a structure to yield a triple bond or at the inner position in a structure to produce three covalent bonds, i.e. one single and one double or three singles. By including carbon, originally with four unpaired electron Lewis structure, the concept is also relevance to organic molecules or ions. Four covalent bonds may be formed by carbon: one single and one triple, two doubles, two singles and one double, or four singles. The four-covalent-bond C, three-covalent-bond N/O+, two-covalent-bond O/S/N-, and one-covalent-bond H/X/O-/S- can be combined in a constant composition with different arrangements to produce isomeric structures of organic molecules4-8 or ions. A summary of atoms commonly found in polyatomic species along with the type and number of covalent bonds each formed is shown in Table 1.
CONCLUSION
Based on the illustration above, it is clear that the proposed procedure of writing Lewis structures is a straightforward approach. The charge for a polyatomic ion with surrounding H/X is assigned only to a central atom. For ions with surrounding O/N/S, each charge is assigned to each O followed by N and S (step1). A single, double, triple bond is then written for each surrounding H/X/O-/S-, O/S/N-, N/O+ on a central atom (step 2). Both steps could be carried out simultaneously. The number of lone pairs on central atom is equal to the number of its valence electrons minus the number of covalent bonds, in pairs (step 3). Most multiple-bonded structures should be modified to produce an all single-bonded structure, or one of resonance structures that will give rise to one or two other resonance structures (step 4). The concepts also can be applied to larger polyatomic molecules or ions of an inorganic or organic origin. Traditionally, many students proceed from inorganic to organic chemistry, or vice-versa, without linking both. The approach outlined here may enable students to link both inorganic and organic chemistry when writing Lewis structures. The skill acquired can be used as an important tool9 to predict geometry,10- 19 physical properties,20,21 as well as reactivity22 of chemical compounds.
REFERENCES