Ultrahigh-current-density niobium disulfide catalysts for hydrogen evolution
Journal: Nature Materials, Impact Factor 38.9 (click here for full paper)
Author: Jieun Yang, Abdul Rahman Mohmad, Yan Wang, Raymond Fullon, Xiuju Song, Fang Zhao, Ibrahim Bozkurt, Mathias Augustin, Elton J. G. Santos, Hyeon Suk Shin, Wenjing Zhang, Damien Voiry, Hu Young Jeong & Manish Chhowalla
Contact details for further info: armohmad@ukm.edu.my
Summary:
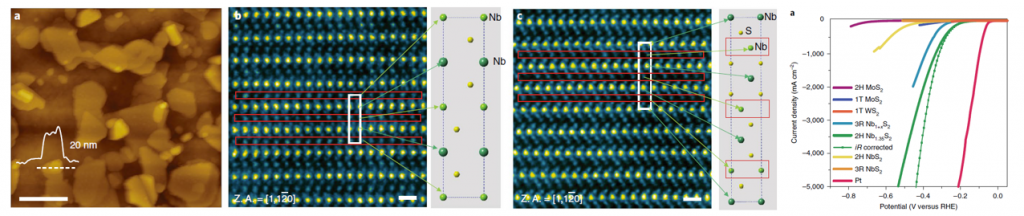
Metallic transition metal dichalcogenides (TMDs) are good catalysts for the hydrogen evolution reaction (HER). The overpotential and Tafel slope values of metallic phases and edges of two-dimensional (2D) TMDs approach those of Pt. However, the overall current density of 2D TMD catalysts remains orders of magnitude lower (~10–100 mA cm−2) than industrial Pt and Ir electrolysers (>1,000 mA cm−2). Here, we report the synthesis of the metallic 2H phase of niobium disulfide with additional niobium (2H Nb1+xS2, where x is ~0.35) as a HER catalyst with current densities of >5,000 mA cm−2 at ~420 mV versus a reversible hydrogen electrode. We find the exchange current density at 0 V for 2H Nb1.35S2 to be ~0.8 mA cm−2, corresponding to a turnover frequency of ~0.2 s−1. We demonstrate an electrolyser based on a 2H Nb1+xS2 cathode that can generate current densities of 1,000 mA cm−2. Our theoretical results reveal that 2H Nb1+xS2 with Nb-terminated surface has free energy for hydrogen adsorption that is close to thermoneutral, facilitating HER. Therefore, 2H Nb1+xS2 could be a viable catalyst for practical electrolysers.